What is Usher Syndrome?
Usher syndrome is the most common genetic cause of combined deafness and blindness. Usher syndrome (USH) is a rare inherited condition - passing from parents to children - that impacts three major senses in the body: hearing, vision, and balance.
There are three clinical types of Usher syndrome: Type 1 usually causes profound deafness at birth, vestibular (balance) dysfunction, and progressive vision loss due to retinitis pigmentosa; Type 2 usually causes moderate to severe hearing loss at birth and progressive vision loss; Type 3 usually causes later onset progressive hearing loss and progressive vision loss. Some individuals with Type 3 also experience balance issues. Usher syndrome is estimated to affect at least 25,000 people in the United States and over 400,000 worldwide.
There is currently no treatment for the hearing, vision, and balance issues associated with Usher syndrome, but there is a growing USH community. Building a community leads to education, support, and treatment.
-
6,173
-
2,908
What is the Usher Syndrome Coalition?
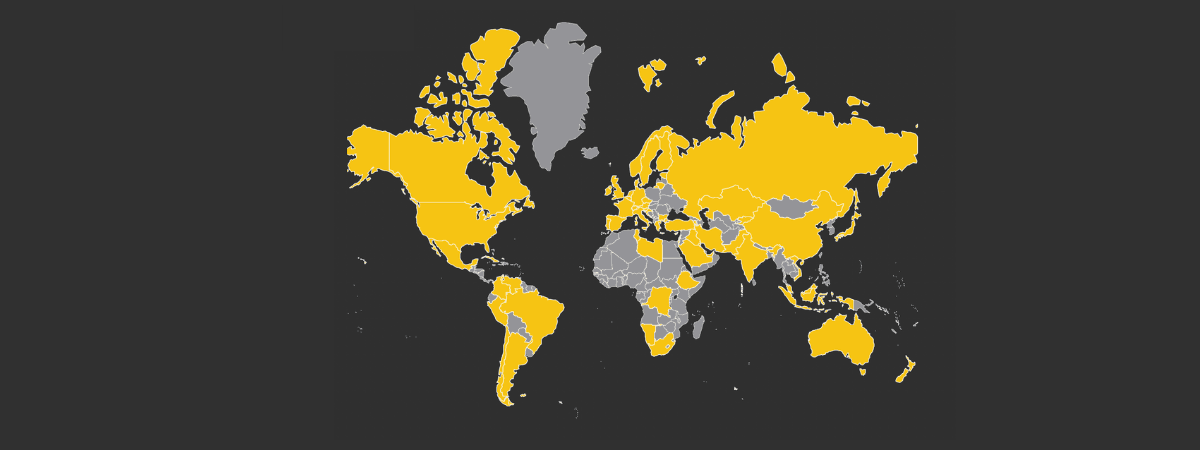
The Usher Syndrome Coalition is the only organization in the world working to find and support every individual and family living with USH, regardless of where they live, what type of USH they were born with, or their method of communication. Our mission is to raise awareness and accelerate research while providing information and support to individuals and families affected by Usher syndrome. We strive to be the most comprehensive resource for the Usher syndrome community, bridging the gap between researchers and families. Learn more and get involved.
The Usher Syndrome Coalition: connecting the global Usher community.
Usher Syndrome Blog & News
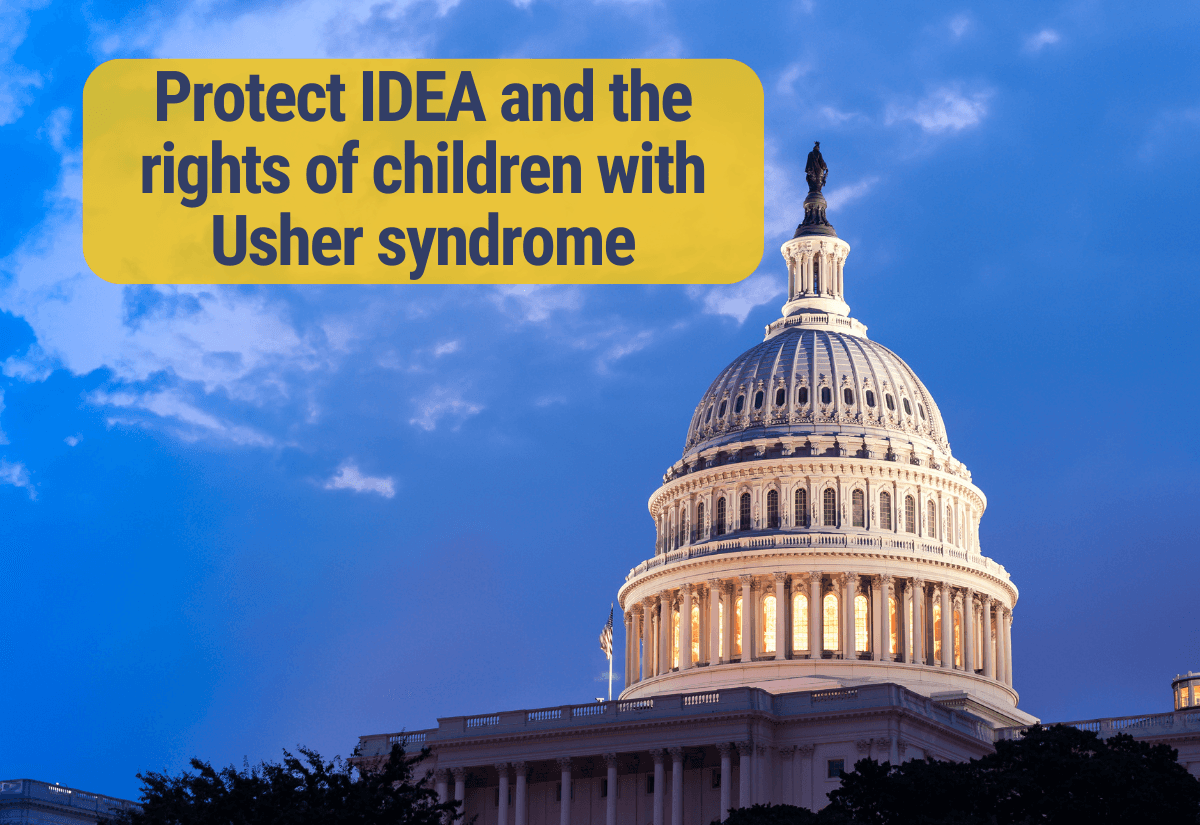
The Usher Syndrome Coalition urges bipartisan, bicameral oversight hearings about the Executive Branch's current and expected actions to illegally restructure the Department of Education, including the Office of Special Education and Rehabilitative Services (OSERS). History has taught us that leaving the enforcement of civil/educational rights laws up to certain states and districts can harm vulnerable communities, especially children with disabilities.
AAVantgarde is working on a new gene therapy and has launched a clinical trial for people living with Usher syndrome type 1B.
The Usher Syndrome Coalition is proud to announce a partnership with Eli Lilly and Company (Lilly) and Akouos, a hearing research company, to support clinical trial enrollment for a potential therapy for USH3A-related hearing loss.
You are not alone.

-
Your USH Family
"Earlier this year I was surprised with an Usher diagnosis. It was a relief to find the USH Coalition. They showed me there is a ton of research going on for potential treatments and that I'm not "one in a million" like my doctor made me out to be. I have a community waiting for me with open arms whenever I'm ready."