SLO-RP: Oral dosage treatment for retinitis pigmentosa in Usher syndrome
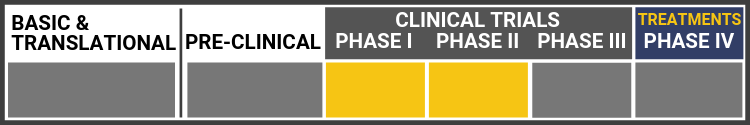
In preclinical animal studies, NPI-001 preserved photoreceptor cells and functionality. A Phase 1 clinical trial of Nacuity’s GMP-grade NPI-001 solution in healthy volunteers was completed with no serious adverse events.
A randomized, placebo-controlled, double-masked, multicenter, Phase 1/2 clinical trial of Nacuity’s proprietary NPI-001 tablets, the SLO-RP Study, is enrolling patients with RP associated with Usher syndrome (NCT04355689). Nacuity expects to report interim safety results from this study before the end of 2022 and interim efficacy results in 2023. The protocol has been amended to allow continued treatment for interested participants while the core study is ongoing.
This product has been granted orphan drug designation. Thus, an eligible new drug application would result in 7 years of U.S. FDA regulatory exclusivity for Nacuity.
Nacuity Related Science News
Nacuity Pharmaceuticals has shared exciting news about a potential new treatment for people with Usher syndrome. They tested an antioxidant tablet called NPI-001 to see if it could slow down vision loss.
Nacuity Pharmaceuticals, Inc. announced that its investigative therapy, NPI-001, for treating retinitis pigmentosa (RP), has received both Fast Track Designation and Orphan Drug Designation from the U.S. FDA.
In June 2023, Nacuity announced they completed enrollment in the SLO-RP Phase I/II clinical trial of NPI-001 tablets.